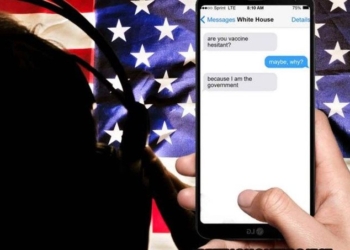
Gov’t to “Engage” SMS Carriers to ‘Dispel Vaccine Misinformation’ by Vetting Your Text Messages
Biden allied groups, including the Democratic National Committee, are also planning to engage fact-checkers more aggressively and work with SMS ...
Read moreDetails